New Drug Designations - February 2024
Shots:
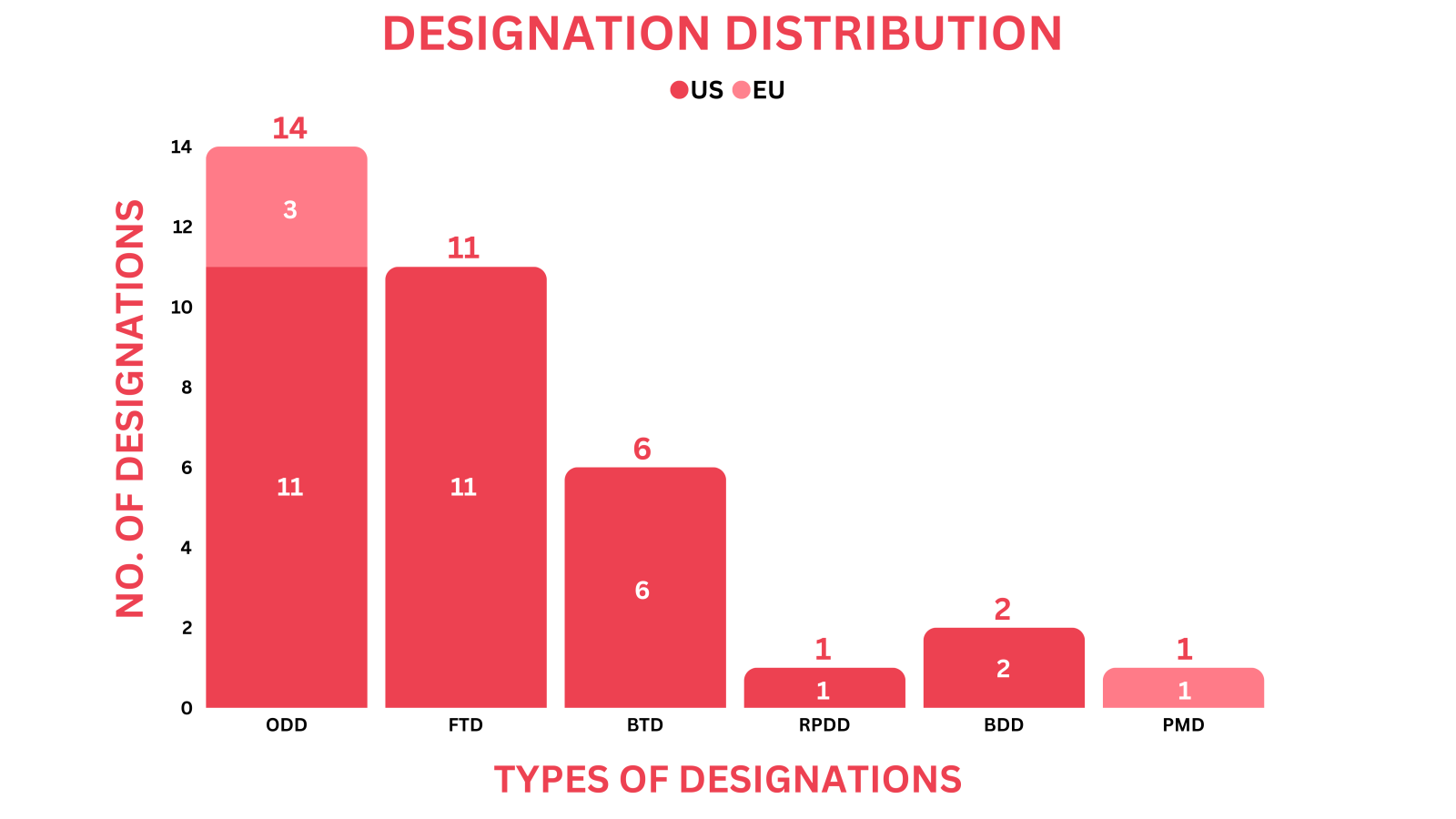
- PharmaShots' designation report provides a concise overview of several drugs and their designations by the US FDA, and EC. This month’s report includes designations allotted to 13 small molecules, 20 biologics, and 2 devices
- SN Bioscience’s SNB-101 received ODD from the US FDA for the treatment of pancreatic cancer based on preclinical results. It has previously received the US FDA’s ODD for treating SCLC in Jul 2023
- PharmaShots has compiled a list of a total of 33 drugs and 2 devices awarded with designations by multiple regulatory bodies in Feb 2024

Olezarsen
.png)
- Ionis Pharmaceuticals’ Olezarsen has received the US FDA's ODD for the treatment of Familial Chylomicronemia Syndrome (FCS)
- Olezarsen is an RNA-targeted LIgand Conjugated Antisense (LICA) drug, it functions by inhibiting the production of apoC-III, which regulates triglyceride metabolism in the blood
LUT014
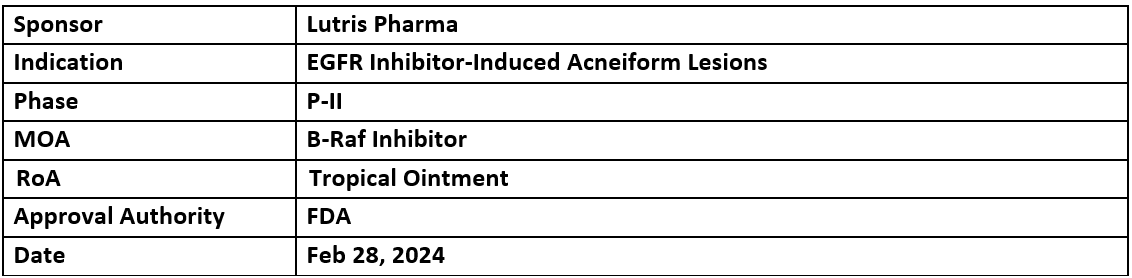
- Lutris Pharma’s LUT014 received the US FDA's ODD for the treatment of EGFR inhibitor-induced acneiform lesions
- P-II study is ongoing in randomized patients. Although, the results P-I trial showed safety and efficacy in colorectal cancer patients who developed grade 2 skin rashes induced by EGFR inhibitorLUT014 (topical application) functions by activating the MAPK pathway to generate new cells and balance cell destruction
Pitolisant
.png)
- Harmony Biosciences’ Pitolisant has received orphan drug designation (ODD) from the US FDA for the treatment of Prader-Willi syndrome (PWS).
- Pitolisant is currently being evaluated in a P-II study (NCT04257929) and is planned to initiate the P-III TEMPO study in Q1’24 for the evaluation of the safety and efficacy of Pitolisant in treating EDS and behavioral disturbances in PWS (age 6+ years).
- Pitolisant is an H3 receptor antagonist/inverse agonist developed by Bioprojet. Harmony holds an exclusive license to develop, manufacture, and commercialize Pitolisant in the US
CardiolRx
.png)
- Cardiol Therapeutics’ CardiolRx has received ODD from the US FDA for the treatment of pericarditis, including recurrent pericarditis. The designation was supported by the pre-clinical and initial clinical data of P-II study
- CardiolRx is currently being evaluated in the P-II (MAvERIC-Pilot) study for recurrent pericarditis and acute myocarditis. The P-II study is evaluating the tolerance, safety, and effects of CardiolRx in patients with recurrent pericarditis and evaluating improvements
- CardiolRx (cannabidiol) inhibits the activation of the inflammasome pathway, an intracellular process important in the development and progression of inflammation and fibrosis associated with myocarditis, pericarditis, and heart failure
Ruxoprubart
.png)
- NovelMed’s Ruxoprubart received ODD from the US FDA for the treatment of Paroxysmal Nocturnal Hemoglobinuria (PNH).
- Ruxoprubart is currently being evaluated for safety and efficacy in a P-II study with treatment-naive PNH patients. The FDA has also approved a P-Ib/II study for PNH, C3 Glomerulopathy (C3G), Atypical Hemolytic Uremic Syndrome (aHUS), and most recently, for ANCA vasculitis (AAV).
- Ruxoprubart is a humanized anti-Bb monoclonal antibody that selectively blocks the alternative pathway while maintaining the functionality of the classical pathway necessary for eliminating infections
Ocifisertib (CFI-400945)
.png)
- Treadwell’s ocifisertib (CFI-400945) has been previously designated with the FTD for the treatment of r/r AML (TP53 mutated) which now received ODD
- The P-Ib/II study is currently evaluating ocifisertib in adult patients with r/r AML and the drug is being administered after the SoC
SNB-101
.png)
- SN Bioscience’s SNB-101 (API: SN-38) received ODD from the US FDA for the treatment of pancreatic cancer based on preclinical results. It has previously received the US FDA’s ODD for treating SCLC in Jul 2023
- The preclinical results demonstrated the drug’s efficacy as compared to Abraxane & Onivyde (existing 1L treatments) in animal models for pancreatic cancer
- Additionally, in Nov 2023, the company received approval to conduct P-II for SNB-101 in Korea. The approval for P-I study has been received in the US & Korea while planning to conduct P-II studies in H2’24 across the US and the EU
IO-202
.png)
- Immune-Onc's IO-202 received ODD from the US FDA for the treatment of CMML and previously received FTD for the treatment of relapsed or refractory CMML in 2023. Furthermore, it was designated with FTD and ODD for AML in 2022 and 2020 respectively
- IO-202 is under the P-I dose expansion study in combination with AZA for the evaluation of safety, PK & PD in patients with newly diagnosed CMML
- IO-202 is a LILRB4 inhibitor and a humanized IgG1 Ab that functions by killing LILRB4hi cells via ADCC and ADCP and can be indicated for the treatment of blood cancers, autoimmune, and inflammatory diseases
Tigilanol Tiglate
.png)
- Qbiotics’s Tigilanol Tiglate received ODD from the US FDA for the treatment of soft tissue sarcoma
- P-II study is ongoing to evaluate efficacy and safety of intratumoral tigilanol tiglate in patients with a range of advanced and/or metastatic soft tissue sarcoma
- The 1EPs is achieved when its ablation rate reduces to >30% tumor volume assessed by ultrasound vs baseline. The 2EPs is to evaluate AEs and serious adverse events and PK
DISC-3405
.png)
- Disc Medicine's DISC-3405 has received the US FDA's ODD for the treatment of Polycythemia Vera (PV).
- DISC-3405 is currently being evaluated in a Phase I study since Oct 2023, and the company plans to develop DISC-3405 initially as a treatment for PV, as well as other hematologic conditions.
- DISC-3405 is an anti-TMPRSS6 (Transmembrane Serine Protease 6, also known as Matriptase-2) monoclonal antibody that functions by increasing the production of hepcidin and suppressing serum iron levels.
Soquelitinib (CPI-818)
.png)
- Corvus' Soquelitinib has received ODD from the US FDA for the treatment of T cell lymphoma (TCL).
- Soquelitinib, which has shown positive interim results from a P-I/IB study in patients with refractory TCL, is expected to advance into a P-III trial in patients with relapsed peripheral T cell lymphoma (PTCL) in Q2’24.
- Soquelitinib selectively inhibits ITK and has the potential to control the differentiation of normal T helper cells, enhancing immune responses to tumors by augmenting the generation of cytotoxic killer T cells and the production of cytokines that inhibit cancer cell survival.
AIT-101
.png)
- OrphAI Therapeutics', AIT-101, has received Orphan Drug Designation from the EMA for the treatment of amyotrophic lateral sclerosis (ALS) and was already designated with the ODD by the US FDA in Jun 2023.
- AIT-101 has recently completed a Phase IIa clinical study in amyotrophic lateral sclerosis (ALS).
- AIT-101 is a potent and highly selective inhibitor of the lipid kinase PIKfyve. This inhibition leads to the activation of the transcription factor TFEB, which drives increased clearance of toxic protein aggregates via the lysosomal autophagy pathway.
NXC-201
.png)
- The European Commission (EC) designated the drug for Orphan Drug Designation (ODD) for AL amyloidosis, while the US FDA had already designated ODD for both AL amyloidosis and multiple myeloma in Sep 2023
- NXC-201 is currently undergoing evaluation in an ongoing Phase Ib/IIa NEXICART-1 (NCT04720313) trial involving patients with multiple myeloma and AL amyloidosis.
- NXC-201 (HBI0101) is an investigational autologous CAR-T therapy designed to target B-cell maturation antigen (BCMA)
ARCT-032
.png)
- Arcturus's ARCT-032 has received Orphan Medicinal Product Designation from the EC based on the positive opinion of the EMA for Cystic Fibrosis (CF) and was already designated with the ODD by the US FDA in Nov 2023
- ARCT-032 is currently being evaluated in a P-Ib study, with the first patient enrolled and administered two doses of ARCT-032. Arcturus expects to share the interim data of the P-Ib study in H1'24
- ARCT-032 utilizes Arcturus' LUNAR lipid-mediated aerosolized platform to deliver CFTR messenger RNA into the lungs and decrease the effects that cause the progressive lung disease

BXCL701
.png)
- BioXcel’s BXCL701 + CPI received FTD from the FDA for the treatment of metastatic small cell neuroendocrine prostate cancer (mSCNC) with progression on CT and no evidence of microsatellite instability, and previously the BXCL701 received ODD in 2019
- BXCL701 is being evaluated for the rendering of “cold” and “hot” tumors making them more detectable by additive immune system therefore developing a strong anti-cancer immune response
- BXCL701 is an oral innate immune activator that functions by initiating inflammation in the tumor microenvironment
Bepirovirsen
.png)
- GSK’s Bepirovirsen received FTD from the US FDA for the treatment of Chronic Hepatitis B (CHB)
- The FTD was granted based on the results from the P-IIb trials incl. (B-Clear) evaluating the safety, efficacy & durability of Bepirovirsen vs PBO in patients (n=440) with chronic HBV infection randomized as those on stable NA (Cohort 1)/ or not (Cohort 2) & (B-Sure) follow-up study evaluating patients achieving a complete or partial response in the (B-Clear) study
- Bepirovirsen, an antisense oligonucleotide (ASO), is currently being evaluated in a confirmatory P-III (B-Well) trial
Vepdegestrant
.png)
- The Arvinas and Pfizer's Vepdegestrant (ARV-471) received the US FDA's FTD for the treatment of ER+/HER2- Breast Cancer in patients who have already received prior endocrine-based therapy.
- The companies are currently evaluating Vepdegestrant in an ongoing P-III (VERITAC-2) study in patients with locally advanced or metastatic ER+/HER2- breast cancer who have already received prior endocrine-based therapy.
- Vepdegestrant is a PROTAC protein degrader that has already shown 97% ER degradation in tumor cells and increased anti-tumor activity versus standard of care (fulvestrant), both as monotherapy and in combination with a CDK4/6 inhibitor
9MW2821
.png)
- Mabwell Bioscience's 9MW2821 has received the US FDA's Fast Track Designation for advanced, recurrent, or metastatic esophageal squamous cell carcinoma (ESCC).
- 9MW2821 is currently being evaluated in an ongoing Phase II study, as indicated by preliminary data showing an objective response rate (ORR) of 30% and disease control rate (DCR) of 73.3% at a dose of 1.25 mg/kg in 30 patients with advanced EC who have already undergone monotherapy and completed at least one tumor assessment.
- 9MW2821 is a Nectin-4-targeting drug developed using an ADC platform and an automated high-throughput hybridoma antibody discovery platform.
EDG-5506
.png)
- Edgewise Therapeutics’ EDG-5506 has received FTD from the US FDA for the treatment of Duchenne muscular dystrophy. It has also been granted ODD for both Duchenne and Becker muscular dystrophies, Rare Pediatric Disease Designation (RPDD) for Duchenne, and FTD for Becker.
- Edgewise is currently evaluating EDG-5506 in a P-II study (LYNX) for Duchenne in children aged 4-9 years, as well as in another P-II study (FOX) involving children and adolescents.
- EDG-5506 is a small molecule that prevents contraction-induced muscle damage in dystrophinopathies. The drug acts by selectively reducing injurious mechanical stress caused by the absence of functional dystrophin, thereby allowing for a greater range of muscle contraction
UV1
.png)
- Ultimovacs's UV1 has received Fast Track Designation (FTD) by the US FDA for unresectable malignant pleural mesothelioma to improve overall survival (OS), supported by the results of the Phase II (NIPU) clinical trial presented at the ESMO Congress 2023. UV1 previously received ODD from the US FDA in 2023.
- UV1 is currently being evaluated in a Phase II study comparing UV1 + ipilimumab and nivolumab versus ipilimumab and nivolumab as second-line treatment, following first-line treatment with platinum-based chemotherapy. The results have shown improvement in OS with no observed toxicity.
BST02
.png)
- The US FDA has granted FTD to the Biosyngen’s BST02 for treating multiple types of liver cancer such as hepatocellular carcinoma and cholangiocarcinoma
- BST02 is currently being evaluated in a P-I/II dose escalation & expansion study for its safety, tolerability, and initial efficacy for the treatment of locally advanced/metastatic liver cancer
- BST02 is a T-cell therapy that employs the patient's own tumor infiltrating lymphocytes and has the potential of overcoming distance constraints because of its cryopreserved form and the reduced need for high interleukin-2 dosage
DISC-0974
.png)
- Disc Medicine's DISC-0974 received US FDA's FTD for the treatment of NDD-CKD and anemia.
- Disc is currently conducting P-I studies in patients with myelofibrosis and anemia and in patients with NDD-CKD and anemia.
- DISC-0974 is a mAb that targets a BMP-signaling co-receptor called hemojuvelin (HJV) and functions by suppressing the production of hepcidin, increasing the levels of serum iron in patients suffering from anemia of inflammation
IDP-023
.png)
- The US FDA grants Fast Track Designation (FTD) to Indapta Therapeutics for IDP-023 for the treatment of patients with non-Hodgkin’s lymphoma and multiple myeloma.
- Indapta is now recruiting patients for Phase I trials of IDP-023 and will receive up to three planned doses of IDP-023 with or without interleukin-2
AlloNK (AB-101)
.png)
- Artiva Biotherapeutics’ AlloNK + rituximab or obinutuzumab received FTD by the US FDA for the treatment of lupus nephritis (LN)
- The P-I/II data showed the ability of AlloNK + rituximab to drive deep B-cell depletion in patients with late-line B-cell cancers
- AlloNK MOA is very similar to the B-cell targeted autologous CAR-T therapies, but it offers the advantage of self-therapy with a better safety profile
PGN-EDODM1
.png)
- PepGen’s PGN-EDODM1 has received the US FDA’s FTD for the treatment of Myotonic Dystrophy Type 1 (DM1) and it was already designated with ODD last year.
- PepGen is currently conducting the P-I study (FREEDOM-DM1) to evaluate PGN-EDODM1 in patients with DM1 and expects to report the initial data this year.
- PGN-EDODM1 functions by delivering a peptide-conjugated antisense oligonucleotide (ASO) for the restoration of cellular function

NVL-520
.png)
- Nuvalent’s NVL-520 received BTD from the US FDA to treat ROS1-Positive mNSCLC and was supported by the P-I part of the P-I/II ARROS-1 clinical trial.
- NVL-520 is currently being evaluated in a P-I/II ARROS-1 trial for patients with solid tumors, including advanced ROS1-positive NSCLC.
- NVL-520 is a brain-penetrant ROS1 Selective Inhibitor designed to act on tumors that have developed resistance to currently available ROS1 inhibitors. Furthermore, it aims to enhance brain penetration to address brain metastases and to avoid inhibition of structurally related TRK.
Sibeprenlimab
.png)
- Otsuka and Visterra’s Sibeprenlimab received the US FDA's BTD for the treatment of immunoglobulin A nephropathy.
- Sibeprenlimab is currently evaluated in P-III study and the companies has reported promising results in P-II ENVISION trial for IgAN in Nov 2023
- Sibeprenlimab (VIS649) is a humanized IgG2 mAb that decreases the production of Gd-IgA1 by binding to specific signaling molecule called APRIL (A ProlifeRation-Inducing Ligand), which has been demonstrated to be a driver of IgA and Gd-IgA1 production
Nipocalimab (mAb)
.png)
- J&J's Nipocalimab received BTD from the US FDA for the treatment of Hemolytic Disease of the Fetus and Newborn (HDFN)
- BTD was backed by P-II (UNITY) study that has met the primary endpoint with minimal adverse events. The P-II (UNITY) clinical trial is a non-blinded study to evaluate the safety, efficacy, PK, and PD of nipocalimab in pregnant participants with EOS HDFN
- Nipocalimab is currently evaluated in a P-III (AZALEA) pivotal trial enrolling pregnant patients who are at risk for severe HDFN and who have a history of severe HDFN in a prior pregnancy(ies)
- Nipocalimab is a mAb that functions by selectively blocking the FcRn to decrease the levels of IgG Ab, incl. auto-Ab and allo-Ab that cause various conditions
Latozinemab (AL001)
.png)
- Alector and GSK's Latozinemab received BTD from the US FDA for the treatment of Frontotemporal Dementia with a progranulin gene mutation (FTD-GRN) in patients
- The BTD was supported by data from the Phase II (INFRONT-2) study of Latozinemab in FTD-GRN patients. Latozinemab is currently being evaluated in a Phase III (INFRONT-3) study in FTD-GRN patients
- Latozinemab (AL001) is a monoclonal antibody that modulates progranulin (PGRN), which is linked to various neurodegenerative disorders. The drug aims to increase PGRN levels in humans by inhibiting sortilin
AlphaMedix (212Pb-DOTAMTATE)
.png)
- RadioMedix and Orano Med’s AlphaMedix (212Pb-DOTAMTATE) received BTD from the US FDA for the treatment of adults with unresectable/metastatic, progressive somatostatin receptor-expressing GEP-NETs who are not treated with peptide receptor radionuclide therapy (PRRT).
- The designation was supported by P-I data, demonstrating an ORR of 62.5%, and an ongoing P-II study investigating the safety and efficacy of AlphaMedix. The target response rate has been attained in the P-II, while top-line results are anticipated in mid-2024
- AlphaMedix comprises SSTR-targeting peptide complex radiolabeled with 212Pb, which produces alpha particles for destroying cancer cells while reducing toxicity to nearby healthy tissues
BAY 2927088
.png)
- Bayer’s BAY 2927088 received BTD from the US FDA for the treatment of NSCLC patients with age ≥18yrs.
- The BTD was granted based on the results from the P-I clinical trial evaluating the safety, efficacy & PK of BAY 2927088 in NSCLC patients (n=460, age ≥18yrs.) with HER2 mutations & who have received prior systemic therapy
- BAY 2927088 is an oral reversible TKI that functions by inhibiting the mutant HER2 incl. HER2 exon 20 insertions & HER2 point mutations & EGFR. The product was developed under the collaboration of Bayer & Broad Institute of MIT and Harvard

AOC 1044
.png)
- Avidity Biosciences’ AOC 1044 was previously designated as ODD and FTD by the US FDA and ODD by the EMA
- P-I/II EXPLORE44 is a randomized, placebo-controlled study to evaluate the safety, tolerability, PK, and PD effects of single and multiple ascending doses of AOC 1044 in healthy individuals and patients with DMD44
- AOC 1044 functions by delivering phosphorodiamidate morpholino oligomers (PMOs) to skeletal muscle and heart tissue to specifically skip exon 44 of the dystrophin gene to initiate the production of dystrophin in people living with DMD44

ARC-BCI System
.png)
- ONWARD Medical’s ARC-BCI System has received the US FDA’s BDD based on the results from two feasibility clinical studies.
- ARC-BCI System utilizes brain-computer interface (BCI) technology + ARC-IM therapy to restore thought-driven lower limb mobility after spinal cord injury (SCI). The company anticipates the launch of its first product in 2024.
- The implanted BCI in the system captures brain activities and signals indicating movement intentions that are decoded into instructions using AI. Afterward, the system stimulates the injured spinal cord to restore thought-driven movement after paralysis
Cognitive Behavioral Therapy (CBT)
.png)
- Better Therapeutics' CBT platform has received the US FDA's BDD to treat adults with MASH/NASH.
- The company has received BDD status on the LivVita study results and meets its 1EPs and 2EPs by reducing liver fat within 90 days and improving liver health without any device-related AEs respectively.
- In 2023, based on clinical results on Type 2 Diabetes (T2D), Better's CBT platform led to FDA authorization of AspyreRx, the first prescription digital therapy delivering CBT as a treatment for T2D

GTX-102
.png)
- GTX-102, which is already designated with ODD by EMA, is now assigned with PRIME Designation for treating pediatric patients with Angelman Syndrome (AS). Furthermore, the drug has already been designated with ODD, RPDD, and FTD by the US FDA.
- PRIME Designation is supported by early clinical data from P-I/II extension arms showing meaningful improvements in several neurodevelopmental domains in AS patients
- P-I/II is a multiple-dose, dose-escalating study to evaluate the safety and tolerability of GTX-102. Patients in earlier extension arms (4-7) transitioned to long-term maintenance dosing, and enrollment for a new expansion arm has been completed to determine the dose range and treatment regimen to be used in P-III study.
- GTX-102 is an oligonucleotide that functions by targeting and inhibiting the expression of UBE3A-AS. Preclinical studies have shown that it stimulates UBE3A expression and decreases the level of UBE3A-AS
Related Post: New Drug Designations - January 2024
Tags

Disha is a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.